Answer:
B) Na3BO3.
Step-by-step explanation:
Hello!
In this case, since percent compositions are used to identify the empirical formula of an unknown compound, we can assume we have 54.0 g of sodium, 8.50 g of boron and 37.5 g of oxygen, and we compute the moles of each one:
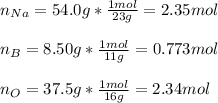
Now, we divide by the moles of boron as those are the fewest:
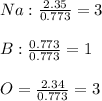
Thus, the empirical formula is B) Na3BO3.
Best regards!