Answer:
a) [H₃O⁺] = 1.8x10⁻⁵ M
b) pH = 4.75
c) % rxn = 3.5x10⁻³ %
Step-by-step explanation:
a) The dissociation reaction of HCN is:
HCN(aq) + H₂O(l) ⇄ H₃O⁺(aq) + CN⁻(aq)
0.5 M - x x x
The dissociation constant from the above reactions is given by:
![Ka = ([H_(3)O^(+)][CN^(-)])/([HCN]) = 6.17 \cdot 10^(-10)](https://img.qammunity.org/2021/formulas/chemistry/high-school/3cq8v17cinwquqdlg8vh7qd7tlyk9uohcf.png)
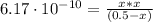
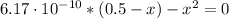
By solving the above quadratic equation we have:
x = 1.75x10⁻⁵ M = 1.8x10⁻⁵ M = [H₃O⁺] = [CN⁻]
Hence, the [H₃O⁺] is 1.8x10⁻⁵ M.
b) The pH is equal to:
![pH = -log[H_(3)O^(+)] = -log(1.75 \cdot 10^(-5) M) = 4.75](https://img.qammunity.org/2021/formulas/chemistry/high-school/i18saedbghmvrk8fmoy3wsuhp0enlq1mc0.png)
Then, the pH of the HCN solution is 4.75.
c) The % reaction is the % ionization:
![\% = (x)/([HCN]) * 100](https://img.qammunity.org/2021/formulas/chemistry/high-school/7m8lvs0hcrde1hxjd20rwf8jlnubaho3mo.png)
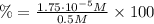
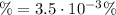
Therefore, the % reaction or % ionization is 3.5x10⁻³ %.
I hope it helps you!