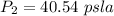Answer:
The value is
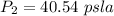
Step-by-step explanation:
From the question we are told that
The initial pressure is
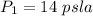
The initial temperature is
![T_1 = 50 \ F = (50 - 32) * [(5)/(9) ] + 273 = 283 \ K](https://img.qammunity.org/2021/formulas/physics/high-school/w6h4ezuimxjckj6rlr874rbfc7f55xg509.png)
The final temperature is
![T_2 = 320 \ F = (320 - 32) * [(5)/(9) ] + 273 =433 \ K](https://img.qammunity.org/2021/formulas/physics/high-school/tdvrvv43qppcj9wmaptwpf4b7qyzvc7uig.png)
Generally the equation for adiabatic process is mathematically represented as
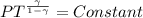
=>
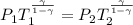
Generally for a monoatomic gas
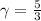
So
![14 * 283^{((5)/(3) )/(1- [(5)/(3) ]) } =P_2 * 433^{((5)/(3) )/(1- [(5)/(3) ]) }](https://img.qammunity.org/2021/formulas/physics/high-school/4mhtyo03fl4f5hj62ersod9a0igr9xehr3.png)
=>
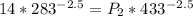
=>