Given :
Volume of gas in 20°C is 50 cm³.
To Find :
The new volume at 96°C.
Solution :
Let, us assume that the pressure is constant throughout the process.
We know, when pressure is constant volume is directly proportional to the temperature.
So,
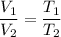
Putting all values in above equation, we get :
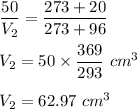
Hence, this is the required solution.