Answer:
Option A
250 degrees Celcius
Step-by-step explanation:
If 1046J of heat energy is added to water, the water will experience a rise in temperature, at a rate that is directly proportional to its specific heat capacity.
Mathematically, this can be seen as
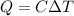
Where C = specific heat of water = 4.184 J/g • °C.
Q = heat energy = 1046 J
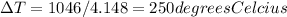
Therefore, the increase in temperature that will be experienced, is for 250 degrees Celcius