Answer :
0.301 moles
Further explanation
Avogadro's hypothesis:
In the same T,P and V, the gas contains the same number of molecules
So the ratio of gas volume will be equal to the ratio of gas moles
Given
n₁=initial mol of Helium : 0.42
V₁=initial volume : 9.9 L
V₂=final volume : 17 L
Required
number of moles added
Analysis
Use formula :
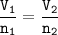
Solution
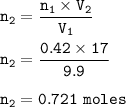
moles added :
0.721 - 0.42 =0.301 moles
Paraphrase
0.301 moles must be added to inflate the balloon to 17.0 L