Answer:
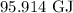
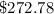
Step-by-step explanation:
m = Mass of water = 749511.5 kg
c = Specific heat of water = 4182 J/kg ⋅°C
 = Change in temperature =
= Change in temperature =
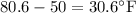
Cost of 1 GJ of energy = $2.844
Heat required is given by
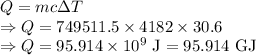
Amount of heat required to heat the water is
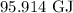 .
.
Cost of heating the water is
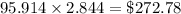
Cost of heating the water to the required temperature is
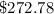 .
.