Answer:
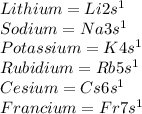
Step-by-step explanation:
When it comes to electron configuration and orbitals, it's important to first identify what exactly we are trying to identify. Below is a given example:
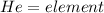
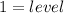
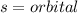
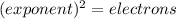
Looking at the periodic table, identify the alkali metal family on the periodic table, or group one elements:
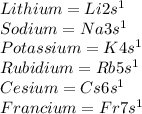
Notice how each configuration has an exponent of
 , representative of a single electron in their s-orbital.
, representative of a single electron in their s-orbital.