Answer:
2.41 * 10^24 atoms of carbon present in the products.
Step-by-step explanation:
First, we need to find the moles of carbon in the products in order to find the number of atoms.
We see that in the chemical formula, carbon belongs to the compound known as glucose. To get the amount of moles of carbon, we need to divide my the amount of carbon within glucose (in grams) from the total amu ( or grams) of glucose to get the moles.
C6 = 72.066.
C6H12O6 = 180.156
72.066/180.156 = 0.4000199827 moles of Carbon within the Glucose compound
Now we will convert to atoms/molecules by multiplying the moles by avogadro's number..
0.4000199827 moles C * 6.022*
 = 2.408920336 *
= 2.408920336 *
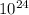 atoms of Carbon present in the products
atoms of Carbon present in the products
*Note --> this can be estimated to 2.41 *
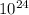 atoms of Carbon
atoms of Carbon