Answer:
The volume of the gas becomes three times the initial volume.
Step-by-step explanation:
Given that the pressure is constant, and temperature changes from -173degree C to 27degree C.
So, the initial temperature,
 = -173 degree C = -173+273 = 100 K.
= -173 degree C = -173+273 = 100 K.
The final temperature,
 = 27 degree C = 27+273=300 K.
= 27 degree C = 27+273=300 K.
As the pressure is constant, so
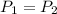 .
.
Let V_1 and V_2 be the initial and final volume respectively.
Assuming that the given gas is ideal gas.
So, applying the ideal gas equation
PV=nRT
where n is the number of moles of the gas and R is the universal gas constant.
For the initial state,
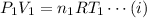
and for the final state,
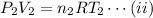
Dividing the equation (i) by (ii), we have
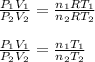
As the mass of the gas is not changing, so
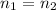 , then
, then
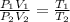
As the pressure is not changing, so
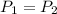 , then
, then
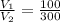
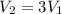
So, the volume of the gas becomes three times the initial volume.