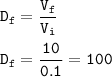The dilution factor : 100
Further explanation
The dilution factor(DF) : ratio of concentration of stock solution and diluted solution or ratio of the volume of final solution to the initial volume from stock solution
From equation of dilution :
M₁V₁=M₂V₂
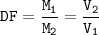
Volume of aliquot = 0.1 ml
Volume of diluent = 9.9 ml
So final volume :

The dilution factor (DF) :