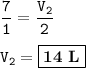You can make 14 L of Ammonia
Further explanation
Avogadro's hypothesis:
In the same T,P and V, the gas contains the same number of molecules
So the ratio of gas volume will be equal to the ratio of gas moles
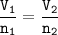
Reaction
N₂+3H₂⇒2NH₃
Volume of Nitrogen-N₂ : V₁=7 L
From the equation above, mol ratio of N₂ : NH₃ =1 : 2, so volume of NH₃ :