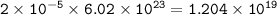There are 1.204 x 10¹⁹ atoms O
Further explanation
The empirical formula is the smallest comparison of atoms of compound forming elements.
A molecular formula is a formula that shows the number of atomic elements that make up a compound.
1 mol of molecule Na₂C₂O₄ :
There are 4 mol of O in 1 mol of molecule Na₂C₂O₄
So for 5 x 10⁻⁶ mol Na₂C₂O₄ :
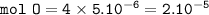
1 mole = 6.02.10²³ particles , so number of atoms O :