Given that,
Volume of gas, V₁ = 200 mL
Initial temperature, T₁ = 27°C
Final temperature, T₂ = -33°C
To find,
New volume of the gas.
Solution,
Initial temperature, T₁ = 27°C = 300 K
Final temperature, T₂ = -33°C = 240 K
Let V₂ is the new volume.
The relation between volume and temperature is given by :
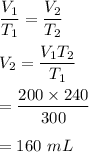
Hence, the volume will be 160 mL.