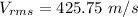Complete Question
Argon gas (a monatomic gas) is sometimes used to insulate a double-pane window for purposes of insulation. In a particular window, the space in between two glass sheets has a volume of
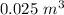 . If the temperature of the gas is 290 K. what is the rms speed of the gas atoms? The atomic mass of Argon is 39.9 u.(l u= 1.66x10-27 kg) Enter your answer in m/s
. If the temperature of the gas is 290 K. what is the rms speed of the gas atoms? The atomic mass of Argon is 39.9 u.(l u= 1.66x10-27 kg) Enter your answer in m/s
Answer:
The value is
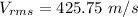
Step-by-step explanation:
From the question we are told that
The volume of the space in between the two glass sheet is
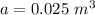
The temperature of the gas is
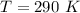
The atomic mass of Argon is

Generally the root mean square velocity is mathematically represented as
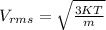
Here K is the Boltzmann constant with value
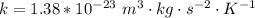
So

=>