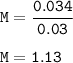The molarity of a solution : 1.13 M
Further explanation
Molarity shows the number of moles of solute in every 1 liter of solute or mmol in each ml of solution
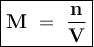
Where
M = Molarity
n = Number of moles of solute
V = Volume of solution
mass HCl = 1.25 g
mol HCl(MW=36.5 g/mol) :
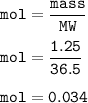
Volume of solution = 30 cm³ = 0.03 L
The molarity :