Answer:
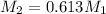
Step-by-step explanation:
 = Concentration of stock solution
= Concentration of stock solution
 = Concentration of solution
= Concentration of solution
 = Volume of stock solution = 19 mL
= Volume of stock solution = 19 mL
 = Volume of solution = 0.31 L= 310 mL
= Volume of solution = 0.31 L= 310 mL
We have the relation
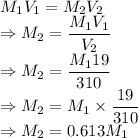
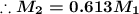
The concentration of the diluted solution will be 0.613 times the concentration of the stock solution.