Answer:
Efficiency = 0.273 = 27.3%
Step-by-step explanation:
The ideal energy that can be produced by certain mass of diesel fuel is given as follows:
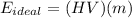
where,
HV = Heating Value of Diesel = 42 - 46 MJ = 44 MJ (Taking average value)
m = mass of diesel fuel = 0.3 kg
E(ideal) = Ideal energy produced by this mass of fuel = ?
Therefore,
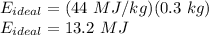
So, the efficiency can be given as:
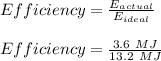
Efficiency = 0.273 = 27.3%