Answer:
No. of Molecules = 18.06 x 10²³ molecules
No. of Atoms of P = 3 atoms
No. of Atoms of Na = 9 atoms
Total No. of Atoms = 24 atoms
Step-by-step explanation:
FOR NUMBER OF MOLECULES:
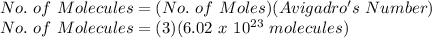
No. of Molecules = 18.06 x 10²³ molecules
FOR NUMBER OF P ATOMS:
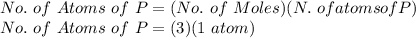
No. of Atoms of P = 3 atoms
FOR NUMBER OF Na ATOMS:
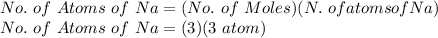
No. of Atoms of Na = 9 atoms
FOR TOTAL NUMBER OF ATOMS:
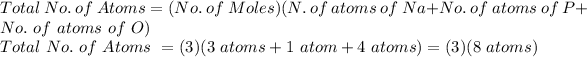
Total No. of Atoms = 24 atoms