Answer:
25 mL
Step-by-step explanation:
The reaction that takes place is:
- Ca(OH)₂ + 2HNO₃ → Ca(NO₃)₂ + 2H₂O
First we calculate how many Ca(OH)₂ moles were spent in the titration:
- 25.0 mL * 0.100 M = 2.5 mmol Ca(OH)₂
Then we convert Ca(OH)₂ moles into HNO₃ moles, using the stoichiometric ratio:
- 2.5 mmol Ca(OH)₂ *
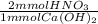 = 5.0 mmol HNO₃
= 5.0 mmol HNO₃
Finally we calculate the volume of required nitric acid solution, using the concentration:
- 5.0 mmol ÷ 0.200 mmol/mL = 25 mL