Answer:
B. One reactant replaces an element in another reactant.
Step-by-step explanation:
Chemical reactions occur when reactants bond or break apart to form new substances known as the products.
Types of Reactions
There are a few main types of reactions:
- Synthesis
- Decomposition
- Single Replacement
- Double Replacement
All of these are different ways chemical reactions can occur.
Synthesis
Synthesis occurs when two different reactants form one product. This would be a match for answer choice A.
- An example includes:
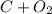 →
→
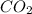
Decomposition
Decomposition occurs when one reactant breaks down, usually through heat, to form 2 different products, which matches answer choice D.
- One example is: NaCl → Na + Cl
Single Replacement
Single replacement occurs when there are 2 reactants, a substance and an element, and the singular elements replaces one of the elements in the substance to form new products. Since this matches answer choice B, this is the correct answer.
- For example, K + NaCl → KCl + Na
Double Replacement
Finally, double replacement is when there are 2 reactants and each switches an element with the other. This is seen in answer choice C.
- One example includes:
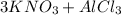 →
→
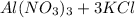
For this example remember that
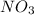 is a polyatomic ion and acts as a singular element.
is a polyatomic ion and acts as a singular element.