Answer:
The mixture of Brand X has a higher concentration of vinegar
Explanation:
Concentration
The concentration of a solution measures the amount of solute that has been dissolved in a given amount of solution. The question states the white vinegar is the solute and water is the solvent to produce the mix or solution.
The concentration can be calculated with the formula:
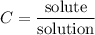
Brand X has 4 ml of white vinegar for every 20 ml of water. The total solution is 4 + 24 = 24 ml. Thus:
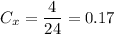
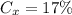
Brand Y has 5 ml of white vinegar for every 30 ml of water. The total solution is 5 + 30 = 35 ml. Thus:
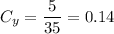
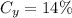
The mixture of Brand X has a higher concentration of vinegar