The electron configuration for Oxygen : [He] 2s²2p⁴
Further explanation
Writing electron configurations starts from the lowest to the highest sub-shell energy level. There are 4 sub-shells in the shell of an atom, namely s, p, d, and f. The maximum number of electrons for each sub-shell is
• s: 2 electrons
• p: 6 electrons
• d: 10 electrons and
• f: 14 electrons
Charging electrons in the sub-shell uses the following sequence:
1s², 2s², 2p⁶, 3s², 3p⁶, 4s², 3d¹⁰, 4p⁶, 5s², 4d¹⁰, 5p⁶, 6s², etc.
The element is Oxgen, with symbol O, and :
the atomic number=8=number of electron
the atomic mass=16
The electron configuration based on the number of electrons(for Oxygen=8), so the configuration :
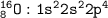 or we can write with noble gas [He] 2s²2p⁴
or we can write with noble gas [He] 2s²2p⁴