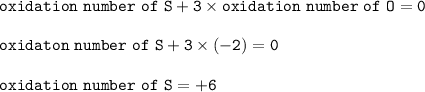The oxidation number for S in the compound SO₃ : +6
Further explanation
The formula for determining Oxidation Numbers :
1. Single element atomic oxidation number = 0.
Group IA (Li, Na, K, Rb, Cs, and Fr): +1
Group IIA (Be, Mg, Ca, Sr and Ba): +2
H in compound = +1, except metal hydride compounds (Hydrogen which binds to groups IA or IIA) oxidation number H = -1, for example, LiH, MgH₂, etc.
2. Oxidation number O in compound = -2, except OF₂ = + 2 and in peroxide (Na₂O₂, BaO₂) = -1 and superoxide, for example KO₂ = -1/2.
3 The oxidation number in an uncharged compound = 0,
Total oxidation number in ion = ion charge, Example NO₃⁻ = -1
The oxidation number for S in SO₃ :