Given that,
Work done by the system = 125 J
Energy released when it cools down = 438 J
To find,
The change in internal energy.
Solution,
As heat is released by the system, Q = -438 J
Work done by the system, W = -125 J
Using the first law of thermodynamics. The change in internal energy is given by :
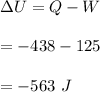
So, the change in internal energy is 563 J.