Answer:
Lithium = 8%
Bromine = 92%
Step-by-step explanation:
To calculate percent composition, you must:
- Calculate the molar mass.
- Divide the subtotal for each element's mass by the molar mass.
- Convert to a percentage
With that being said, given LiBr (Lithium Bromide), calculate the molar mass:
Lithium has an atomic weight of 7, and there is one Lithium atoms in LiBr. Bromine has an atomic weight of 80, and there is one Bromine atom in LiBr:
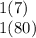
Add the two products:
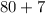
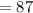
The molar mass of LiBr is about 87 grams.
With that information, divide the subtotal of Lithium by the molar mass, and then multiply by 100 to convert to a percentage:
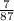 ×
×
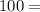
8.0459%
(Round to nearest percentage):
8%
Therefore, the percent composition of Lithium in the compound Lithium Bromide is about 8%.
Now, divide the subtotal of Bromine by the molar mass, and then multiply by 100 to convert to a percentage:
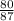 ×
×
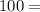
91.9540
(Round):
92%
Therefore, the percent composition of Bromine in the compound Lithium Bromide is about 92%.