Answer:
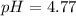
Step-by-step explanation:
Hello!
In this case, since the initial acetic acid and its conjugate base are mixed, we need to compute the new concentration via the final volume (100 mL):
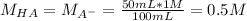
Which means the concentration of the acid and conjugate base are the same, therefore, the pH of the solution equals the pKa of the acid:
![pH=pKa+log(([A^-])/([HA]) )\\\\pH=-log(1.7x10^(-5))+log((0.5M)/(0.5M) )\\\\pH=4.77+0\\\\pH=4.77](https://img.qammunity.org/2021/formulas/chemistry/college/igaa3kl3la9h56gb88ykosf5t48bvgvag8.png)
Best regards!