Answer:
Rate of internal heat transfer = 23.2 Btu/Ibm
mass flow rate = 21.55 Ibm/s
Step-by-step explanation:
using given data to obtain values from table F7.1
Enthalpy of water at temperature of 100 F = 68.04Btu/Ibm
Enthalpy of water at temperature of 50 F = 18.05 Btu/Ibm
from table F.3
specific constant of glycerin
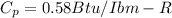
The rate of internal heat transfer ( change in enthalpy )
h4 - h3 = Cp ( T4 - T3 ) --------------- ( 1 )
where ; T4 = 50 F
T3 = 10 F
Cp = 0.58 Btu/Ibm-R
substitute given values into equation 1
change in enthalpy ( h4 - h3 ) = 23.2 Btu/Ibm
Determine mass flow rate of glycol
attached below is the detailed solution
mass flow rate of glycol = 21.55 Ibm/s