Answer:

Step-by-step explanation:
First, find the molar mass of calcium (Ca). This can be found on the Periodic Table.
Next, find the number of moles.
Avogadro's Number tells us the number of atoms in one mole.
Therefore, there are 6.023*10²³ atoms in 1 mole.
We have 6.023*10²³ atoms of calcium, so it must also be 1 mole of calcium.
Finally, find the mass. Set up a proportion using the molar mass of calcium.
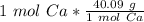
Multiply. The moles (mol Ca) will cancel.
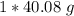
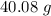
There are 40.08 grams in 6.023*10²³ atoms of calcium.