Answer:
(Q1 - Q2)/Q1
Step-by-step explanation:
The efficiency of any device can be given by the following formula:
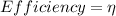 = Output/Input
= Output/Input
Now, for engine the output is the mechanical work that it does and the input is the heat that it absorbs. Let:
W = Work Done by the engine
Q₁ = Heat Absorbed by the System
Q₂ = Heat Rejected by the System (Because According to 2nd Law of Thermodynamics, engine can not convert the entire heat into work without rejection of some heat)
Therefore, the equation becomes:
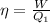
but, the work can be written as the difference between the absorbed heat and the rejected heat. Hence,
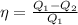
Therefore, the correct option is:
(Q1 - Q2)/Q1