Answer:
4 Sb, 3

Step-by-step explanation:
On the reactant's side of the equation (the left side), there is one Antimony and one Oxygen gas molecule (
 ). The oxygen gas molecule is made of two atoms, so we actually have 2 oxygens on the left side. On the product's side (the right side), there are 4 antimony atoms, and 6 oxygen atoms. If we were to write it out in a certain way, it would look like this:
). The oxygen gas molecule is made of two atoms, so we actually have 2 oxygens on the left side. On the product's side (the right side), there are 4 antimony atoms, and 6 oxygen atoms. If we were to write it out in a certain way, it would look like this:
__Sb + __
 -->
-->
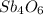
1 Sb 4
2 O 6
To balance this equation, those numbers on either side of the elements must equal each other. We can accomplish this with the proper coefficients. If we put a 4 in front of the antimony, it means this:
4 Sb + __
 -->
-->
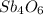
4 Sb 4
2 O 6
And the antimony is now balanced.
Now we must balance the oxygen. There are 6 oxygens on the product's side but only 2 on the reactant's side. To fix this, simply multiply the oxygen by 3:
4 Sb + 3
 -->
-->
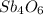
4 Sb 4
6 O 6
3 * 2 = 6, so now oxygen is balanced, and the equation is now correct.