Moles of gas = 0.123
Further explanation
In general, the gas equation can be written
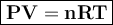
where
P = pressure, atm , N/m²
V = volume, liter
n = number of moles
R = gas constant = 0.082 l.atm / mol K (P= atm, v= liter),or 8,314 J/mol K (P=Pa or N/m2, v= m³)
T = temperature, Kelvin
Volume(V) =2.5 L
Pressure(P) = 1.2 atm
Temperature(T) = 25 + 273=298 K
