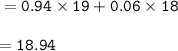The relative mass of the sample : 18.94
Further explanation
The elements in nature have several types of isotopes
Isotopes are atoms whose no-atom has the same number of protons while still having a different number of neutrons.
So Isotopes are elements that have the same Atomic Number (Proton)
Atomic mass is the average atomic mass of all its isotopes
Mass atom X = mass isotope 1 . % + mass isotope 2.%
F-19 = 94% of the sample
F-18 = 100%-94%=6% of the sample
The relative mass :