The empirical formula : C₁₂H₄F₇
The molecular formula : C₂₄H₈F₁₄
Further explanation
mol C (MW=12 g/mol)
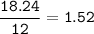
mol H(MW=1 g/mol) :
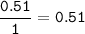
mol F(MW=19 g/mol)
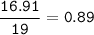
mol ratio of C : H : O =1.52 : 0.51 : 0.89=3 : 1 : 1.75=12 : 4 : 7
Empirical formula : C₁₂H₄F₇
(Empirical formula)n=molecular formula
( C₁₂H₄F₇)n=562 g/mol
(12.12+4.1+7.19)n=562
(281)n=562⇒ n =2
Molecular formula : C₂₄H₈F₁₄