Answer:
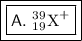
Step-by-step explanation:
Isotope notation is written as:
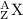
where A is the mass number and Z is the atomic number. A charge can be added to the right subscript if needed.
1. Find A
A is the mass number, or the sum of protons and neutrons.
There are 19 protons and 20 neutrons.
- A= protons+ neutrons
- A=19 + 20 =39
2. Find Z
Z is the atomic number of number of protons.
There are 19 protons.
3. Find a possible charge
If this is a neutral atom, the protons equal the neutrons.
There are 19 protons and 18 electrons, which is obviously not equal. This is an ion and there is a charge.
There is 1 more proton. A proton has a positive charge, so the charge must be +1.
4. Write the symbol.
A= 39, Z= 19, and the charge is +1
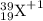
So, the correct answer must be choice A.