The percent yield of the reaction : 89.14%
Further explanation
Reaction of Ammonia and Oxygen in a lab :
4 NH₃ (g) + 5 O₂ (g) ⇒ 4 NO(g)+ 6 H₂O(g)
mass NH₃ = 80 g
mol NH₃ (MW=17 g/mol):
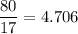
mass O₂ = 120 g
mol O₂(MW=32 g/mol) :
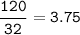
Mol ratio of reactants(to find limiting reatants) :
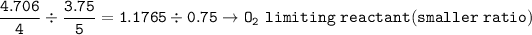
mol of H₂O based on O₂ as limiting reactants :
mol H₂O :
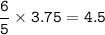
mass H₂O :
4.5 x 18 g/mol = 81 g
The percent yield :
