The molecular formula of this protein : C₁₈H₄₂O₁₂N₆
Further explanation
The empirical formula is the smallest comparison of atoms of compound forming elements.
A molecular formula is a formula that shows the number of atomic elements that make up a compound.
(empirical formula) n = molecular formula
The principle of determining empirical formula and molecular formula
- Determine the mass ratio of the constituent elements of the compound.
- Determine the mole ratio by dividing the elemental mass with the relative atomic mass obtained by the empirical formula
- Determine molecular formulas by looking for values of n
Find mol ratio for every component :
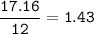
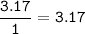
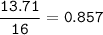
N (r=14 g/mol) :
Mass of Nitrogen :
40.4-(17.16+3.17+13.71)=6.36 g
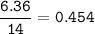
C : H : O : N = 1.43 : 3.17 : 0.857 : 0.454 = 3.15 : 7 : 1.89 : 1=3:7:2:1
Empirical formula : C₃H₇O₂N
Molecular mass of protein :
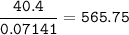
(C₃H₇O₂N)n=565.75
(12.3+1.7+2.16+14)n=565.75
(89)n=565.75
n=6.4≈6
so the molecular formula : C₁₈H₄₂O₁₂N₆