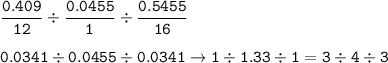The empirical formula for this vitamin : C₃H₄O₃
Further explanation
The empirical formula is the smallest comparison of atoms of compound =mole ratio of the components
The principle of determining empirical formula
- Determine the mass ratio of the constituent elements of the compound.
- Determine the mole ratio by dividing the percentage by the atomic mass
Mass of C in CO₂ :(MW C = 12 g/mol, CO₂=44 g/mol)
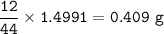
Mass of H in H₂O :(MW H = 1 g/mol, H₂O = 18 g/mol)
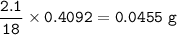
Mass O = Mass sample - (mass C + mass H) :
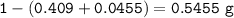
mol ratio C : H : O =