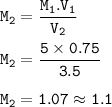The molarity of the diluted solution : 1.1 M
Further explanation
Dilution is the process of adding solvent to get a more dilute solution.
The moles(n) before and after dilution are the same.
Can be formulated :
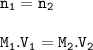
M₁ = Molarity of the solution before dilution
V₁ = volume of the solution before dilution
M₂ = Molarity of the solution after dilution
V₂ = Molarity volume of the solution after dilution
M₁=5
V₁=0.75
V₂=3.5