Mass of NaOH = 223.983 g
Further explanation
A mole is a unit of many particles (atoms, molecules, ions) where 1 mole is the number of particles contained in a substance that is the same amount as many atoms in 12 gr C-12
1 mole = 6.02.10²³ particles
While the number of moles can also be obtained by dividing the mass (in grams) by the molar mass of an element or molecule
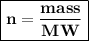
MW of NaOH = 39.997 g/mol
moles of NaOH= 5.6
Mass of NaOH :
