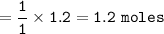Moles of CuS : 1.2
Further explanation
The reaction coefficient in a chemical equation shows the mole ratio of the components of the reactants and products
If one mole of the reactant or product is known, then we can determine the moles of the other compounds involved in the reaction
Reaction
CuCl₂ (aq) + H₂S (g) → CuS (s) + 2HCl (aq)
CuCl are mixed with excess of H₂S gas, so CuCl as a limiting reactant
mol ratio of CuCl₂ : mol CuS = 1 : 1, so mol CuS :