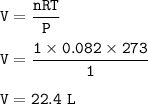The volume of one mole of an ideal gas at STP = 22.4 L
Further explanation
The ideal gas equation can be written
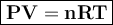
where
P = pressure, atm
V = volume, liter
n = number of moles
R = gas constant = 0.08206 L.atm / mol K
T = temperature, Kelvin
n=1 mol
standard temperature and pressure = T = 273 K, P = 1 atm
The volume :