The percent yield of the reaction : 77.2%
Further explanation
Percent yield is the comparison of the amount of product obtained from a reaction with the amount you calculated
General formula:
Percent yield = (Actual yield / theoretical yield )x 100%
An actual yield is the amount of product actually produced by the reaction. A theoretical yield is the amount of product that you calculate from the reaction equation according to the product and reactant coefficients
Reaction
Cu + 4HNO₃ → Cu(NO₃)₂ + 2NO₂ + 2H₂O
mol of Cu(MW=63.55 g/mol) :
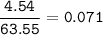
mol ratio Cu : Cu(NO₃)₂ = 1 : 1, so mol Cu(NO₃)₂ :
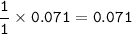
mass of Cu(NO₃)₂ (MW=187,56 g/mol) :
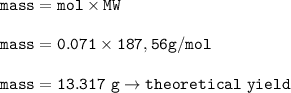
10.28 g of Cu(NO₃)₂ are obtained from the reaction⇒actual yield
The percent yield :
