Answer:
Cesium is an Element in the Periodic table with atomic number 55, symbol Cs and belongs to the first group of the metals which is Alkali metals.
Ionization energy (IE) is defined as the amount of energy consumed by a ground state gaseous atom to discharge an electron which will results in a formation of a Cation
It forms ions be releasing the electron
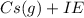 →
→
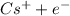 IE= 38.939 KJ
IE= 38.939 KJ