Answer:
Step-by-step explanation:
The formation of ammonia that occurred by the reaction of nitrogen and ammonia is expressed as:
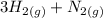 ⇄
⇄
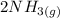
where;
The reactants are:
Hydrogen and nitrogen
The product is ammonia.
For the reaction, the equilibrium constant can be expressed as:
![K = ([product])/([reactants])](https://img.qammunity.org/2021/formulas/chemistry/high-school/6h7cqz6w65smyj9tnde0wrnwnv53pa7592.png)
![K = ([NH_3]^2)/([N_2]^3[H_2]^3)](https://img.qammunity.org/2021/formulas/chemistry/high-school/uz0yxisphppmls4tsuthexjgv3hcl8hd22.png)
From the equilibrium constant conditions, the formation of ammonia and its decomposition due to its reversible reaction back to hydrogen and nitrogen are equal. It implies that the rate of the forward reaction is also equal to that of the backward reaction.
Thus, during when equilibrium is obtained;
Hydrogen, Nitrogen, and Ammonia are present.