Answer:
41.43 mL
Step-by-step explanation:
First we calculate the mass of NaOH in the original solution:
- 25.0 % m/v means that in 100 mL of solution, there are 25 grams of NaOH.
- This means that in 5.80 mL, we would have (5.80 * 25/100) 1.45 grams of NaOH.
Then we calculate the volume of the diluted solution, using the grams of NaOH (that remain the same throughout the dilution process):
- 1.45 g NaOH *
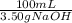 = 41.43 mL
= 41.43 mL