Answer:
V = 6.04 10⁻⁶ V
Step-by-step explanation:
The energy in a system is conserved so the potential energy of the system must be transformed into the kinetic energy of the electron.
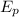 = K
= K
eV = ½ m v²
V = ½ m v²/e
Let's use the de Broglie relation
λ= h / p
the moment is
p = mv
let's replace
λ = h / mv
v =

hence the potential energy
V = ½
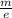 (\frac{h}{m \lambda })²
(\frac{h}{m \lambda })²
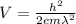
let's reduce λ to sistem SI
λ = 500 nm = 500 10⁻⁹ m = 5 10⁻⁷ m
let's calculate
V = (6.63 10⁻³⁴)² / (9.1 10⁻³¹ 1.6 10⁻¹⁹ (5 10⁻⁷)² )
V = 6.04 10⁻⁶ V