Answer:
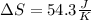
Step-by-step explanation:
Hello!
In this case, for the given reaction, we can write the equation to compute the entropy change as shown below:
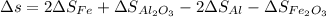
Letting:
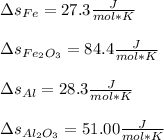
We obtain the entropy change per mole of Fe2O3(s):
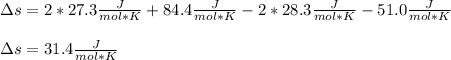
Finally, the total entropy change when 1.73 moles of Fe2O3(s) react turns out:
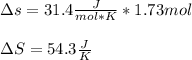
Best regards!