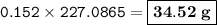The mass of nitroglycerin : 34.52 g
Further explanation
Reaction
4C₃H₅N₃O₉ ⇒ 12 CO₂ + 10H₂O + 6N₂ +O₂
Volume = 10 L
Temperature = -5°C=268 °K
Pressure = 1 atm
mol of CO₂ (ideal gas) :
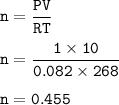
mol ratio C₃H₅N₃O₉ : mol CO₂= 4 : 12, so mol C₃H₅N₃O₉ :
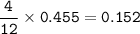
mass C₃H₅N₃O₉ (MW=227,0865 g/mol):