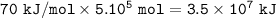Heat energy needed : 3.5 x 10⁷ kJ
Further explanation
Heat is a form of energy that can flow from high-temperature objects to low-temperature objects.
So heat moves when there is a difference in temperature and can increase or decrease the temperature. An object that receives a lot of heat will cause a large increase in temperature
The amount of heat is influenced by the mass of the object and the difference in temperature
Can be formulated
Q = m.c.Δt
Q: heat received or removed by an object (J)
m: object mass (kg)
c: heat type substance (J / kg⁰C)
ΔT: temperature change (⁰C)
210 kJ of heat energy is used to form 3.0 moles of Hydrogen, so heat energy for 1 mol Hydrogen :

mol of 1000 kg of Hydrogen=10⁶ g(MW=2 g/mol)
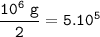
The heat energy :